Resulting Concentration Of Solution (m)
V2 is the final volume of the diluted solution. Molarity is measured in number of moles of a substance per unit volume.
Weightvolume percentage concentration is usually abbreviated as wv To calculate wv concentration.

Resulting concentration of solution (m). How do you know how much of the stock solution to use and how much of the pure solvent to use. Solute the dissolved material and solvent the liquid in which the solute is dissolved. To dilute a solution means to add more solvent without the addition of more solute.
25 10 3 L 060 m o l L 1 0050 L 0025 L 0200 m o l L 1. 811 Percent by mass mass of solute mass of solution 100 Suppose that a solution was prepared by dissolving 250 g of sugar into 100 g of water. This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration.
The concentration of stock solution is 05000 M. Molarity of the resulting solution M 0. 4 m o l e s.
An alternative and commonly-used notation for this equation is M1V1 M2V2 where M is used in place of C. This is the volume that results after V1 from the stock solution has been diluted with diluent to achieve a total diluted volume of V2. We have to use a 5 member calibration seria.
When the solute in a solution is a solid a convenient way to express the concentration is a mass percent massmass which is the grams of solute per 100 g of solution. Each volumetrick flasks are 200 ml. For example if a solution with a concentration of 1 mgmL is diluted to yield a solution with a concentration of 1 gmL the resulting dilution factor is 1000.
Assuming the volumes are additive calculate the molarity of the second HCl solution. Concentration of Solutions Recall that a solution consists of two components. The resulting solution contains the amount of solute originally taken from the stock solution but disperses that solute throughout a greater volume.
We have to prepare a calibration the concentration of the solutions has to be between 10-4 M and 10-3 M. The units of molar concentration are moles per cubic decimeterThey are noted as moldm as well as M pronounced molar. The resulting solution is 0974 M.
The two most common ways of expressing concentration are molarity and molality. C2 is the final concentration of the diluted solution. The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute eg the concentration of hydroxide anions can be written as OH.
Therefore the final concentration is lower. Concentration Moles of solute Volume of solutionand thus we get units of m o l L 1. 4 1.
Mass of solute g volume of solution mL 100. The final solution is less concentrated and more dilute. Calculate the mole fraction of NaOH.
Of course the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. The amount of solute in a given amount of solution or solvent is known as the concentration. Click hereto get an answer to your question The molar concentration of chloride ions in the resulting solution of 300mL of 30M NaCl and 200 mL of 40MBaCl2 will be.
MOLARITY M Most common way of expressing concentration Molarity can be used to calculate the volume of solvent or the amount of solute. Total number of moles of NaCl 1 0. M1V1 M2V2 M3V3 0445 200 x 388 0974 200 388.
The volume of the resulting solution is 10200 mL. And so we get. SAMPLE PROBLEM A solution prepared by dissolving 400 grams of NaOH in 100 g of water.
Common units for wv concentration are g100mL Solubilities are sometimes given in units of grams of solute per 100 mL of water that is as a weightvolume percentage. 4 m o l e s 2 M. 7 L 1.
Number of moles of NaCl in 2 0 0 ml of 2 M solution 2 1 0 0 0 m l 2 0 0 m l 0. How do you prepare the solutions. For this particular dilution it can also be said that the stock solution was diluted 1000- fold.

How To Calculate Molarity Concentration Persuasive Writing Prompts Word Problem Worksheets Writing Rubric

Image Result For Fluid And Electrolytes Made Easy Nursing School Tips Fluid Electrolytes Pathophysiology Nursing

Pin On Trauma Assessment 1 247974 Tarsha Teague

Solution Concentration Boundless Chemistry

5 Easy Ways To Calculate The Concentration Of A Solution
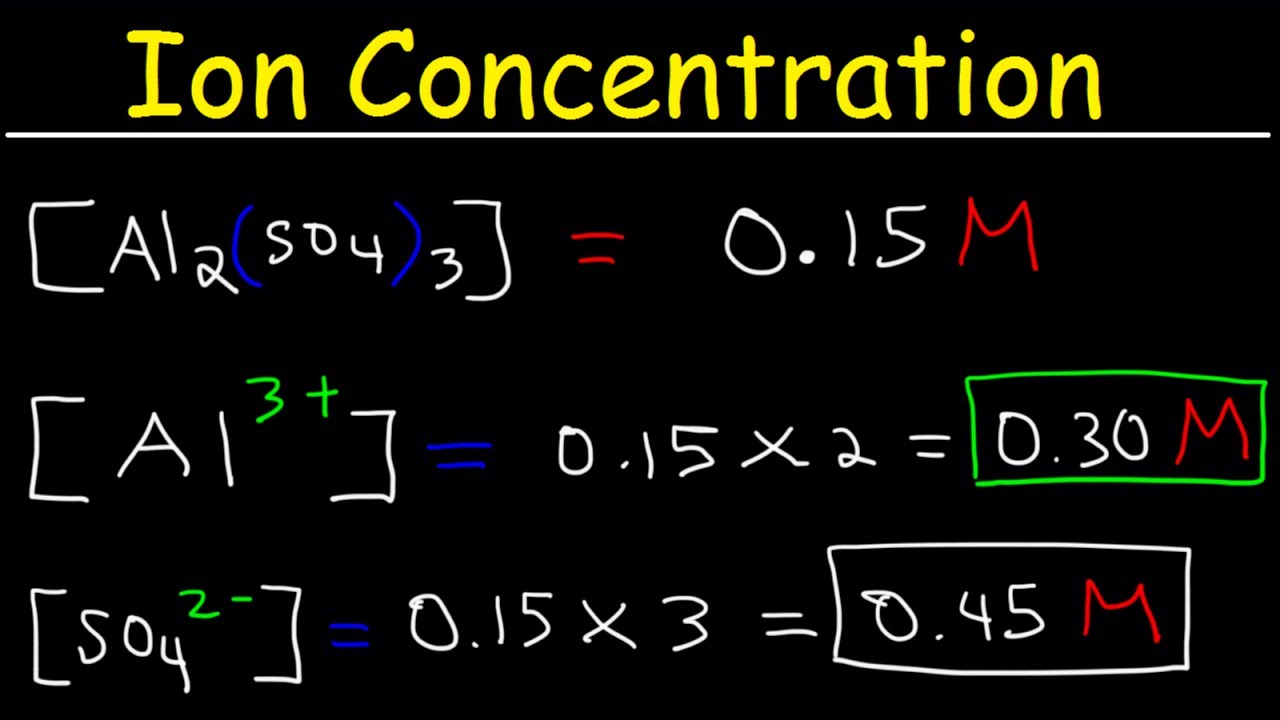
Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube

Pin By Bianca M Rodan And Fields On Redefine In 2020 Wrinkles Retinol Skin

A Practical Approach To Chemical Peels Chemical Peel Aesthetic Dermatology Dermatology

5 Easy Ways To Calculate The Concentration Of A Solution

5 Easy Ways To Calculate The Concentration Of A Solution
Molarity And Solution Units Of Concentration

5 Easy Ways To Calculate The Concentration Of A Solution

5 Easy Ways To Calculate The Concentration Of A Solution

Hierarchically Porous Fiber By Electrospinning And Posttreatment Fiber 3d Printing Technology Innovation Centre

Sel Social Emotional Learning For Kids Teens Adults At The Meeting House Misbehaved Or Sensory Overload Our Ot Speaks Out Sensory Processing Sensory Processing Disorder Processing Disorder





Post a Comment for "Resulting Concentration Of Solution (m)"