What Is Ionic Strength Of A Solution
The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. It represents the variation of activity coefficient with concentration in electrolyte solutions.
How is it calculated.

What is ionic strength of a solution. Ionic strength represents the total electrostatic forces contributed by ions in solution and can be calculated as shown in the following equation. The concentration of a solution is calculated with the strength of the solution by using the formula concentration of a solution solute mass in gramsolution volume in mL x 1000. Ionic strength is the total ion concentration in solution.
Also to know what is the unit of ionic strength. Ionic compounds when dissolved in water dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation.
Be sure to include the concentrations of Ag and Br as well as the concentrations of the ions from the strong electrolyte. Ionic compounds when dissolved in water dissociate into ions. Ionic strength refers to a measure of the concentration of ions present in a particular solution.
The ionic strength of a solution is a measure of the concentration of ions in that solution. When a solution is less concentrated than 001 M is used to determine the activity A of each ion in solution. It was defined by Lewis and Randall in 1921 and it is based on the dissociation that suffers salts acid and bases when are in an aqueous solution.
On dissolving in water they dissociate into ions. However weak acids or bases are only partially dissociated in solution and thus equilibrium is established between ions and the un-dissociated molecules. Calculate the ionic strength for each of the solutions in the table on page 4.
The ionic strength of a solution is a measure of the concentration of ions in that solution. The ionic strength of a solution is a function of the concentration of all ions present in a solution. Strength of solution Mass of solute in gramsVolume of a solution in litres.
A is always inferior to the. Due to the square of z i multivalent ions contribute particularly strongly to the ionic. Ionic strength is a measure of the ions concentration ions solution.
The ionic strength of the sol is directly proportional to the concentration of ionized species. What is ionic strength of a solution calculated. It is expressed in concentration units such as molar concentration molL.
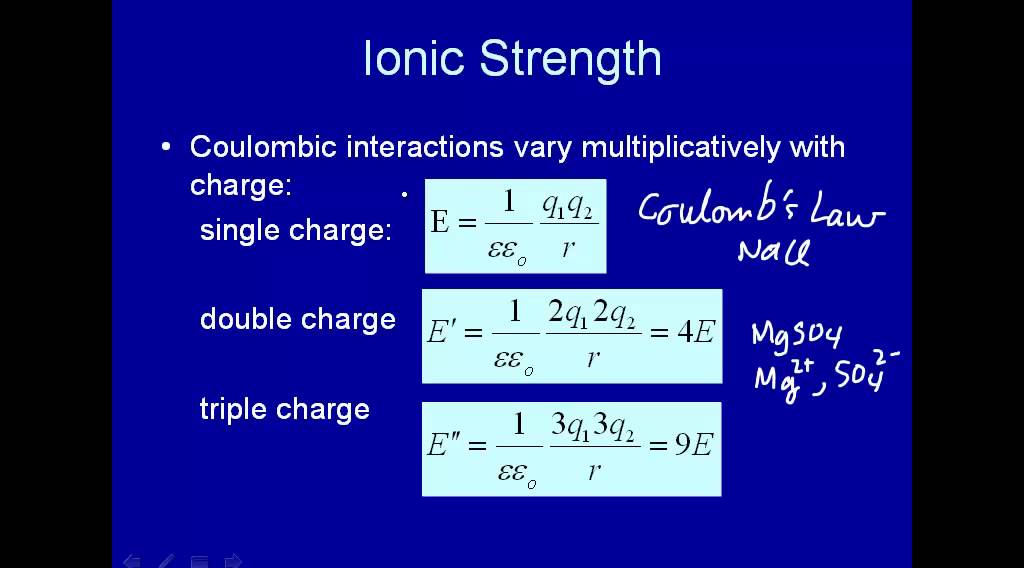
Use this formula to calculate ionic strength. I 12 ni CiZi squared where I represents ionic strength n represents the number of ions in solution i represents the specific ion in solution Ci represents the concentration to the th species such as moles per liter Zi represents the valence or. The unit for ionic strength is molarity.
The ionic strength of the solgel solution is a very important tool for controlling the solgel process. How is it calculated. Feel free to divide the work with other classmates.
Ionic strength is mainly used in the study of not too concentrated solutions of strong electrolytes like all soluble salts NaCl CaBr2 KNO3 and strong acids HCl HBr HClO4 H2SO4 so defined because totally dissociated in solution. What is ionic strength of a solution. You must be signed in to discuss.
The ionic strength of a solution is a calculated using the concentration of dissolved substances in a solution along with the valence of the species in question. 5 large I frac12 sumlimits_iz_i2 c_i Here c i and z i are the molar concentration and the charge of ion i. HA H A Strong acids are considered to be completely dissociated into ions in dilute solution.
Where Ci is the molar concentration of any ion in solution and zi is its valence. What is ionic strength of a solution. What is the effective pH r 0025.
Knowing ionic strength is important to chemists because ions have an electrical charge that attract or repel each other. Ionic compounds when dissolved in water dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation.
Clinical Chemistry - Theory Analysis Correlation 2020. Ionic strength is used to calculate activity and activity coefficient of ions and electrolytes in solution. In typical solgel systems this is determined by the concentration and pK.
What is the ionic strength of the current buffer. Ionic compounds can certainly dissolve in water. Ionic strength represents the relative interactions of ions with water molecules and other ions in a solution.
This attraction and repulsion causes ions to behave in certain ways. Hydrogen ions in the solution arise from the dissociation of acids. The ionic strength of a solution is a measure of the concentration of ions in that solution.
The sum is taken over all ions in the solution. Ionic strength is a measure of the intensity of the electrical field created in a solution by an ionic activity .

Dissociation Dissociation Easy Science Stem For Kids

Pin By Science Solutions On Chemistry Molecules Word Search Puzzle Chemistry

Ph Log H Assuming 100 Percent Dissociation If Given Percent Ionization Multiply By The Molarity Chemistry Education Chemistry Lessons Teaching Chemistry

How To Write Ionic Equations Tutor Pace Science Chemistry Chemistry Class Chemistry Notes

Pin By Maria On Science Chemistry Basics Chemistry Notes Science Classroom

Pin By Science Solutions On Education Chemistry Solutions Education

What Is Neutralization Here Is The Exact Answer Chemistry 8th Grade Science Teaching Science

Ionic Strength And Salt Effects On Binding And Solubility The Bumbling Biochemist

Ionic Strength Solved Problems Iit Jee Neet Jam Csir Net Gate Youtube

C H Bond Is Stronger Than C C Bond Teaching Chemistry Bond Length Chemistry

Molality Formula Chemistry Lessons Study Chemistry Chemistry Basics

Solving Dilution Problems In Solution Chemistry Clear Simple Word Problem Worksheets Math Facts Addition Writing Rubric

Pin By Science Solutions On Education Linear Programming Math Equations Mathematics






Post a Comment for "What Is Ionic Strength Of A Solution"